When Lejla Kameric’s daughter was treated for non-Hodgkin’s lymphoma, the twelve-year-old had to get through lumbar punctures without pain relief. “15 years ago in Bosnia, painful procedures and diagnostic activities were done without anaesthesia,” Kameric recalls. “Still now, young doctors are fighting to use anaesthesia – and only from time to time do they succeed.”
Treatment of childhood cancer is not equal within Europe – and this inequality leaves tangible marks. The most recent study of childhood cancer survival across Europe showed that survival was generally lowest in Eastern Europe, where the survival rate for paediatric cancer was between 10 and 20% lower than in Western Europe. And for cancers with poor outcomes, such as AML or osteosarcomas, these disparities became even larger.
Those figures came from the 2013 Eurocare-5 study, which looked at outcomes for people diagnosed between 1990 and 2007. A more up-to-date picture of pan-European disparities can be gleaned from a 2020 study. looking at childhood cancer mortality trends in Europe between 1990 and 2017. Based on data extracted from the WHO mortality database, the study showed that childhood cancer mortality rates declined remarkably over that time period across Europe (by around 2.8% a year), with rates declining fastest in eastern and central European countries. It also showed, however, serious geographic disparities remain.
The most recent mortality data (2014‒2016) show a 2.5-fold difference between the best and the worst across Europe, with the highest rates still found in central and eastern European countries. So while mortality rates from childhood cancers average 2.28 per 100,000 across North-West-Europe, that rises by more than 50% to an average of 3.56 per 100,000 across Centre-East Europe. The EU countries with the highest mortality rate from childhood cancer are Romania (3.61 per 100,000) and Hungary (3.57 per 100,000).
These averages hide some significant disparities within the broad geographic regions. In the Czech Republic, for instance, the mortality rate from childhood cancer now stands at 2.14 per 100,000 children ‒ better than the average for North-West Europe and topped only by two European countries: Belgium (1.98 per 100,000) and Switzerland (1.72 per 100,000). Limited data availability, however, means that no conclusions could be drawn about survival rates in some eastern European countries, mostly in the Balkan region, including in Bosnia and Herzegovina, Montenegro, and Albania. One indication from the Balkan region comes from Serbia, where the mortality rate is 3.74 per 100,000. Only the Ukraine fared worse in this comparison, with an average rate of 4.25 per 100,000.
The authors conclude that, “Further efforts are required to fill the gap, by promoting widespread and rational adoption of currently available treatment protocols.”
Towards common standards of care
Inequalities arise along the entire spectrum of childhood cancer care, from diagnosis to cancer treatment, supportive care and survivorship or palliative care, says Pamela Kearns, Professor of Clinical Paediatric Oncology at the University of Birmingham, UK, and President of SIOP Europe, the European Society of Paediatric Oncology. “Our ambition as SIOPE is that it should not matter in which part of Europe you are diagnosed, you should have access to the same care, regardless of geography.”
Carmello Rizzari, head of the Paediatric Haematology-Oncology Unit in Monza, Italy, and President-Elect of SIOP Europe, echoes this sentiment. “It is not acceptable that one kid with the same disease in one country in Europe will not have the same possibilities of detailed diagnosis and appropriate treatment as in another country. These inequalities must be fought by all the stakeholders involved in the field of paediatric oncology, and are under the focus of the future SIOPE efforts.”
Efforts to improve access, especially to treatment, are being undertaken by SIOPE, but importantly also by parent organisations and individual initiatives in eastern European countries.
Lejla Kameric now lives in Vienna, but she is still involved in improving care in Bosnia-Herzegovina through the organisation A Heart for Kids with Cancer (Srce za djeku). Kameric also leads the ‘Diagnosis, Treatment and Care’ pillar of CCI Europe, the pan-European organisation of parents whose children have been or are being treated for cancer.
One way in which CCI Europe seeks to even out inequalities is by laying out common standards of care delivery. Already in 2009, CCI Europe and SIOPE published the ‘European Standards of Care for Children with Cancer’. This consensus document defined the minimum standards that institutions should meet when caring for children with cancer, including the standard facilities and the delivery of therapy. Some points make provisions that might appear ‘standard’ for western European countries, such as continuous parental involvement, but were not a given – at least in Bosnia ‒ at the time of publication, Kameric recalls. “We didn’t have access to any information, for example the results of [my daughter’s] blood work, to see if therapy was working or she’d be eligible for a different therapy.”
“We want to check if it is useful, for example as political leverage, to have a document that shows how care should be done”
Now, almost 15 years after the ‘Standards of Care for Children with Cancer’ were published, CCI Europe has launched an initiative to check the implementation across Europe. A relaunch of the publication is also planned, with updates to areas where good progress has been made over the past decade – such as in follow-up care and palliative care. “We’re starting first with the CCI Europe network, and will work with SIOPE on the new version of the document,” says Kameric.
One important section of CCI Europe’s survey will be to assess whether parents find the European Standards of Care helpful for advocating for better care. “We want to check if it is useful for our members, for example as political leverage, to have a document that shows how care should be done,” she says.
Shortages in essential medicines
Political leverage is also one of the goals of a project driven by SIOPE as part of the EU Joint Action on Rare Cancers (JARC): namely assessing access to essential medicines required for treating children with cancer. In this project, led by Gilles Vassal, Professor of Paediatric Oncology at Gustave Roussy in Paris, a list of medicines was drawn up, based on the WHO Model List of Essential Medicines for Children 2017. “We did a deep-dive in the definition of what medicines are essential and should be available across Europe 24/7,” Vassal recalls. This list was sent to healthcare professionals and parent associations in 37 European countries, to assess how available these medicines are.
As a result of this survey, a range of problems that hinder accessibility was identified in a study published this year. These problems include drug shortages, out-of-pocket costs, lack of appropriate formulations, and lack of pain management. Less than two-thirds of medicines on the WHO list were reported to be ‘always available’ in at least 90% of surveyed countries. Five medicines essential for treating acute lymphoblastic leukaemia (ALL) ‒ the most frequent cancer in children ‒ were reported as being ‘always available’ in less than 60% of responding countries. Access to medicines required for controlling side effects was also unreliable; pain control for lumbar puncture was reported as ‘always available’ by less than half of responding parents (48%). The financial burden of cancer remains unequal as well, with almost one-third of responding parents (32%) reporting that they had to pay for their child’s hospital treatment either in part or in full. “There are really inequalities in access to essential medicines,” Vassal sums up.
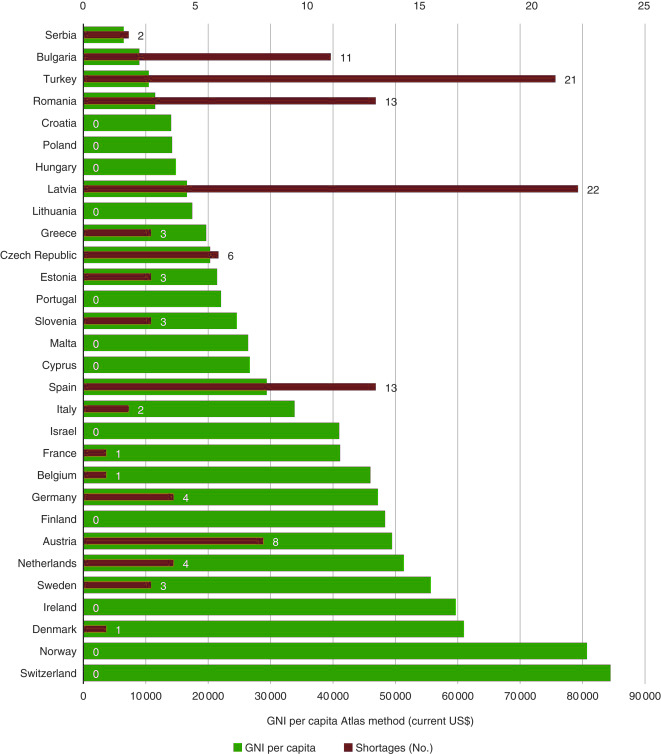
Source: G Vassal et al (2021) Access to essential anticancer medicines for children and adolescents in Europe. Ann Oncol 32:560‒568, Figure 3. Republished under a Creative Commons Licence. © 2021 The Authors. Published by Elsevier Ltd on behalf of European Society for Medical Oncology
For Vassal, this damning evidence should be enough to spur politicians into action. “We tell politicians: This is the reality, we provide you with the evidence, you find the solution.” The reasons behind shortages, such as procurement and supply chains, and the duty and ability to tackle these problems, are beyond the remit of SIOPE, says Vassal. “It’s not my job to find the solution. But my job is to warn people and say: this is the problem. And when children don’t have access to asparaginases for leukaemia, the risk that they die of this exists.” SIOPE is also contributing to an update of WHO’s essential medicines list. “The fact that there is a list of medicines is a message that [countries] should make these available. It’s not enough, but it is a minimum that should be available,” says Vassal.
“We deliver the drug to them – sometimes involving pharmacies in Vienna, and suitcases to Sarajevo… Our goal is that the kid is cured.”
CCI Europe is also active in the essential medicines initiative to reduce inequalities, but Kameric is realistic about its prospects for affecting change. “Does that mean that doctors are obliged to have it? No. But at least that’s one step towards it.” Drug shortages are improving in Bosnia and other countries, Kameric adds, but progress is slow. Located now in the “Old West”, Kameric knows about the tactics sometimes required to get much needed medicine to children. “Methotrexate is a basic drug, but often, it is not available – because of complicated, slow procedures in the country that don’t allow ordering it. But it is important to get the therapy on time, especially with kids. And so we use the opportunity to deliver the drug to them – sometimes involving pharmacies in Vienna, and suitcases to Sarajevo… In the end, our goal is that the kid is cured. It doesn’t matter what is the cost.”
Even when drugs are available, knowledge about best practice treatment can be limited. The challenge here is two-fold, says Pamela Kearns. “Do you put in place in every country, in every geographical site, the expertise that’s needed? Or do you make sure that it is possible for the expertise to be available when needed. I think it has to be a mixed model, with at least one centre of expertise in every country that can provide the best standard of care.”
To make expertise available, SIOPE has been developing the European Standard Clinical Practice (ESCP) project, to define what the ‘best standard of care’ is for common childhood cancers.
In many countries, children are being treated on clinical trial, but when a clinical trial is not available, a guidance on best-practice needs to be available and has been lacking
“We wanted to standardise what would be best-practice treatment, regardless of where you live. We have been defining the right diagnostics for a disease, and what standard treatment should be available to every child.” Again, the guidelines are also intended to give leverage to parents and doctors to demand the provision of these services. “They can say to the healthcare service: This is the best standard and how are you going to ensure we can provide it?”
“The guidelines are also intended to give leverage to parents and doctors to demand the provision of these services”
The guidelines will be made available also to parents through CCI Europe, to help parents in the midst of advocating for their children, says Kameric. “The idea is to have expert parents (or caregivers) who can ‘translate’ the guidelines to parents whose kids are in treatment, in order to better understand the situation, but also to advocate in case the treatments and cure described there don’t exist.” While a first round of guidelines are set to be launched this Autumn, SIOPE will be adding to these, going forward, until all common childhood cancers are covered. These guidelines are a game changer for the community, says Kameric. “We’ve been waiting for these for more than twenty years.”
Exchange expertise, not patients
The expert parents identified as central to disseminating the clinical practice guidelines also act as the national contact points for parents within the European Reference Network for Paediatric Oncology, or ‘ERN PaedCan’. ERNs are an EU initiative that encourages national health systems to cooperate and exchange expertise that might be missing within a given country. One vision of ERNs was to establish ‘virtual advisory boards’ ‒ virtual panels of medical experts that would allow knowledge and expertise to move across borders, rather than patients.
Ruth Ladenstein, Professor of Paediatric Oncology at the Children’s Cancer Research Institute, Vienna, is the coordinator of ERN PaedCan. She acknowledges that the virtual advisory boards have not yet reached their full potential, for technical reasons. “The design of the clinical patient management system, in which cases can be uploaded and discussed in virtual tumour boards, is currently not easily accessible for those who need advice. Although we are giving support, the IT is a painful process, and this is not as desired. In the next years, we hope to have a smarter, quicker way of functioning that is also mobile ready. The idea is good, but technically, it needs further fine-tuning and improvement to really achieve the desired effects.”
To avoid brain drain, Ladenstein envisages ‘twinning’, to allow… for training in the institution in EU expansion countries
ERN PaedCan is, however, encouraging other ways in which expertise may flow, including through the twinning of institutions for training. Training programmes can be tricky, says Ladenstein. “To work in a resource-rich country is tempting, but if we have a brain drain, we really haven’t done the country any good.” To avoid brain drain, Ladenstein envisages ‘twinning’ between institutions, to allow for both short-term educational stays in resource-rich countries, but also training in the institution in EU expansion countries. “This interaction is important, also to exchange ideas between partners.”
What shines through in these discussions is that economics and limited resources are at the source of inequalities: the number of drug shortages reported, for example, tends to be greater in countries with lower gross national incomes. “Obviously, [inequalities] are a political problem, that has to be addressed also from the completely different angle of funding,” Ladenstein acknowledges.
Ladenstein is also a member of the EU Mission Board for Cancer, one of the five missions identified by the European Commission within Horizon Europe, its funding programme for research. The Cancer Mission’s 2020 interim report identified tackling childhood cancer as a ‘cross-cutting action’, while the next report – in the last stages of drafting and approval – will, according to Ladenstein, identify funding possibilities. “These are issues that also the Cancer Mission is pinpointing, that there will have to be special European investment funding going to the expansion countries to bring their healthcare systems up to speed. […] Through the Mission, we are trying to bring a lot of innovation across Europe so that the buy-in also comes from the member states to really go for change management in their countries.”
As Kearns points out, the European Standard Clinical Practice guidelines developed by SIOPE are a benchmark that should be achieved regardless by all European countries. “These should be realistic for all countries to achieve, as we are talking about standard treatment. I don’t think the investment should be prohibitive for any government, given the number of children we’re talking about. I suspect the problem is more recognising the need, and, if it is not possible to implement a therapy, to make sure you have an agreement with another centre or another country and have a pathway to deliver it. It is feasible.”
Vassal adds that, while costly innovative cancer treatments can be of value, this is not always the case, and it is important not to waste resources by using them inappropriately. He proposes a careful, potentially joint, assessment should be made of when to use them – and when not. In such an assessment, “we as physicians provide a clear definition of who should get the medicine and who should not. It is our responsibility to use the right new medicine where they’re really improving [outcomes] for children and avoiding the use when it is not relevant.”
“I’ve never seen an era with so much opportunity for us to engage to change policy… and put childhood cancer on the agenda”
Kearns also sees Europe’s Beating Cancer Plan and its ‘Helping Children with Cancer Initiative’ as one potential driver to bring cancer care to a more equal level across Europe. “I have never seen an era where there’s been so much opportunity for us to engage to change policy, to potentially leverage funding, and put childhood cancer genuinely on the agenda. […] I hope we can ride this wave and implement the promises that have been given over the last 18 months.”
A new hospital for a new system?
One area rife with inequality is the provision of radiotherapy for children. A survey published at the ESMO 2017 Congress showed that access to paediatric radiotherapy is unequal across Europe. In one-quarter of the centres analysed, radiotherapy could not be delivered under anaesthesia, which is needed, at least for younger children, to ensure they don’t move around. Romania is one of the countries where children don’t have access to radiotherapy, as Carmen Uscatu, president of ‘Give Life’ (Daruieste Viata) ‒ a group raising funds and campaigning to transform healthcare in Romania ‒ points out. “Radiotherapy departments are only located in hospitals for adults. But there, you don’t have an anaesthesiologist for children. And even if they can organise this kind of service, they don’t.”
Uscatu’s solution: if they don’t have it, build it. ‘Give Life’ is building the first paediatric oncology and radiotherapy hospital in Romania, financed with private donations. “For the first time, a hospital in Romania will have a radiotherapy department dedicated to children.” The initiative, called #NoiFacemUnSpital (We’re building a hospital), started in 2015, when Uscatu and co-founder Oana Gheorghiu wanted to launch a project to renovate the oncology department at Bucharest’s Marie Curie Hospital ‒ the biggest hospital for children in Romania. “We wanted to renovate the ward, simply to build rooms with private toilets. But this was almost impossible, because the oncology department is on the fourth floor. And so we decided to build another building in the yard of the hospital.”
“For the first time, this will be a place where children can receive all the treatments they need”
Construction of the hospital started in 2018, and the new paediatric oncology and radiotherapy hospital should become operational in the summer of 2022. But the changes are not limited to construction: “For the first time, this will be a place where children can receive all the treatments they need.” Rather than being transferred to different hospitals across the city or country to receive surgery, for example, children should then be able to receive all treatments, including neurosurgery, in one place. “We are working to reorganise the hospital,” Uscatu says, to put the patient at the centre. But this won’t be easy, as hospital funds are currently tied to the number of beds provided. “The system is not built for efficiency. We need to change the way [hospitals] are financed by the National Health Insurance.” To staff the hospital, the organisation worked together with Romanian embassies to bring ex-pat doctors back to the country. “But no doctor wanted to come back to Romania,” Uscatu reports. Instead, they will now work together with hospitals in Italy and Ireland to train specialist staff for the hospital.
In 2019, the Give Life association announced that, in addition to the paediatric oncology and radiotherapy wing, it intends to re-build the entire Marie Curie Hospital. There, space will also be reserved for research, Uscatu explains, which would be essential for participating in clinical trials. “Research is a big problem in Romania, in fact, [clinical] research doesn’t exist. We are not part of the European Union from this point of view.”
A lack of opportunity to be included in clinical trials is a problem when treating children with cancer, as Kearns acknowledges. “When there isn’t a known curative treatment available, [children] need the opportunity to access experimental medicine through clinical trials. If it isn’t available in a centre or a country, the ability to move to where the opportunity to participate in a trial is available needs to be facilitated.” At the moment, participation in clinical trials is not covered by the EU’s cross-border healthcare directive, unlike access to standard treatment. “When [a clinical trial] is the only treatment option available, there should be a provision to allow it to happen.”
Kearns and Rizzari welcome all the recent European initiatives ‒ the European Reference Network, the Cancer Mission and the Beating Cancer Plan ‒ which they say are putting children with cancer and their families under the spotlight, and should help close disparities in care. “But an extraordinary effort is required by all the stakeholders involved, i.e. regulatory bodies, academia, professionals, parents, survivors,” they say. “And within this common effort, SIOPE wants to take all the children with cancer under its wings, and to bring all of them towards an equal environment where diagnosis, treatment, support and follow-up are the same for all children.”
Photo courtesy of SIOPE